Abstract
Introduction: Fanconi Anemia (FA) is a life-threatening genetic disease predisposing patients to bone marrow (BM) failure and neoplasia. The hematolymphoid manifestations of FA can be cured by allogeneic hematopoietic stem cell transplantation (HSCT), however FA patients have high malignancy rates post HSCT due to their underlying defects in DNA damage repair which increase the genotoxicity of chemotherapy and irradiation-based conditioning regimens. Our group has previously developed several monoclonal antibody (mAb)-based conditioning strategies for non-genotoxic HSCT by targeting the HSC-cell surface receptor CD117 which are now at varying stages of clinical development. To translate these approaches to FA, we evaluated the safety and efficacy of different αCD117mAb strategies for HSC depletion and in combination with immunosuppression explored their ability to establish therapeutic donor hematopoiesis post HSCT in FA mice. These strategies would be applicable to treating patients with either allogeneic grafts or autologous genetically-engineered grafts.
Methods: FA mice (Fancd2-/-) were treated with antagonistic αCD117mAb ACK2, combination with inhibitory αCD47mAb MIAP410 or CD117-antibody-drug-conjugate (ADC) 2B8-SAP using previously published protocols (Czechowicz et al. Science 2007; Chhabra et al. Sci Transl Med 2016; Chandrakasan et al. Blood 2017; Czechowicz,* Palchaudhuri* et al. Nat Comm 2019). Safety was assessed by monitoring blood counts/chemistries and histology was performed on various tissues. HSC depletion was assessed phenotypically by flow cytometry and functionally by colony-forming counts (CFCs). FA mice were subsequently conditioned with each of these agents and transplanted with whole BM (WBM) from wildtype C57BL/6N mice. Although, the FA mice had been backcrossed for 10+ generations onto the C57BL/6N background, minor allelic differences were noted when compared to wildtype C57BL/6N mice and hence the depleting αCD4mAb GK1.5 was also given as has been previously published (Chandrakasan et al. Blood 2017). The αCD4mAb was also given alone as a control in addition to an unconditioned group. As HLA-matched donors are not available for all patients, haploidentical transplantation was also modeled in the FA mice using WBM from C57BL/6NxBALB/cAnNCrl F1 donors. Given our prior findings in HLA-matched animals of efficacy with immunosuppression alone conditioning, mice were conditioned with vary immunosuppression regimens without αCD117mAbs (George et al. Cell Stem Cell 2019; Li*, Czechowicz* et al. Nat Comm 2019). Peripheral blood and BM of transplanted mice was monitored for donor chimerism and for functional correction by assessing for resistance to DNA-damaging agent mitomycin-C (MMC).
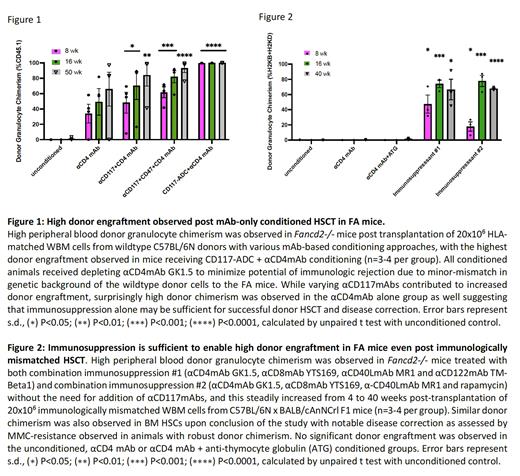
Results: We found that all αCD117mAbs were safe in FA mice with no additional toxicities from those previously reported. Interestingly, we found that HSC depletion and increased donor chimerism were achieved only when αCD117mAbs were augmented with αCD47mAb or drug-conjugation. Surprisingly, FA animals conditioned with αCD4mAb alone showed similar donor chimerism to those conditioned with αCD117mAb + αCD4mAb, with increasing donor chimerism up to >80% suggesting that immunosuppression-only conditioning may be sufficient in FA transplant settings (Fig 1). Similar findings were observed with haploidentical donors, where combination immunosuppression alone was sufficient for high donor engraftment and disease correction (Fig 2). HSC chimerism as assessed on serial BM aspirates also increased with time in FA mice that achieved donor engraftment.
Conclusions: Our novel findings suggest that if sufficient immunosuppression is given to obtain initial donor HSC engraftment in the allogeneic setting, resulting turnover of a majority of the hematolymphoid system can occur likely due to the survival advantage of wildtype HSCs over FA HSCs. Such a non-toxic all antibody-based conditioning strategy could be transformative for FA patients. These promising findings encourage investigating parallel treatment strategies in patients which are being explored in a recently opened clinical trial (NCT03814408), however they also encourage interpretation of results from this trial in the context of these findings. Additionally, these studies suggest the potential of development of immunosuppression-only protocols for FA.
Disclosures
Czechowicz:GV: Consultancy, Current equity holder in publicly-traded company; Jasper Therapeutics: Other: Intellectual Property Rights; STRM.BIO: Research Funding; Teiko Bio: Consultancy, Current holder of stock options in a privately-held company; Rocket Pharmaceuticals, Inc.: Research Funding; Stemodontics: Consultancy, Current holder of stock options in a privately-held company; Editas Medicine: Current equity holder in publicly-traded company; Decibel Therapeutics: Current equity holder in publicly-traded company; Spotlight Therapeutics: Consultancy, Current equity holder in publicly-traded company; Gilead Sciences: Other: Intellectual Property Rights; Beam Therapeutics: Consultancy, Current equity holder in publicly-traded company; Global Blood Therapeutics: Current equity holder in publicly-traded company; Magenta Therapeutics: Current equity holder in publicly-traded company, Other: Intellectual Property Rights.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal